The Value of Clinical Research and Technology Organizations
Clinical Research Organizations (CROs) play a major role in ensuring safe, ethical clinical trials that help spur the development of new, life-saving drugs and medical devices that benefit millions of patients worldwide.
GLOBAL IMPACTS OF CLINICAL RESEARCH
Clinical research and technology organizations have an enormous, positive impact on the drug development process in addition to the American and global economy.
- Each year, ACRO member companies employ more than 130,000 people in over 114 countries – 2x more than in 2008.1
- ACRO members conduct the vast majority of worldwide clinical trials each year, involving nearly two million research participants.1
- Clinical research conducted by CROs increased 40% from 2008-2014,1 and is expected to reach over $45 billion/year by 2022.2
PROMOTING QUALITY
ACRO member companies and the entire clinical research industry help ensure that worldwide standards for clinical research trials are established and maintained. ACRO is also committed to advancing clinical trial outsourcing to improve the quality of biomedical research. That translates to higher-quality clinical trials that spur the development of new drugs while protecting patients and research participants globally.
Worldwide, 76 percent of clinical trials are conducted in either the United States or Europe, with the remainder taking place in Asia, Latin America, Africa, and the Middle East. According to the Drug Information Journal, there were no significant differences in data quality—regardless of where the trials took place. ACRO member companies play a vital role in protecting the quality of medical research trials worldwide.


ENSURING EFFICIENCY
By advancing global clinical trials and the drug development process, ACRO members are committed to improving efficiency in the field of biomedical research. According to the independent Tufts Center for the Study of Drug Development:
- Clinical trials conducted by CROs—like the ones that make up ACRO’s membership—are completed on average 30 days quicker than those conducted by sponsor companies.
When it comes to medical research and the drug development process, time really is money. That’s why clinical research and technology organizations enhance efficiency by encouraging innovation and leading the globalization of clinical research.
ENCOURAGING INNOVATION
ACRO members are developing and using innovative, technology-driven tools to transform clinical research. Decentralized trials, adaptive trials, artificial intelligence and machine learning, risk-based monitoring, new site selection and patient recruitment strategies – all these innovations enhance efficiency and help to bring new, life-saving drugs, treatments, therapies, or medical devices to the market faster than ever before. From drug discovery services, through the development process, and continuing into market access and reimbursement strategies, clinical research and technology organizations are adding value.
ACRO and its member companies will continue to pursue and strengthen relationships with policy makers, regulatory agencies and government officials to ensure the continued progress and advancement of safe, high-quality, innovative clinical trials. We also work to promote tax, trade and business policies that promote innovation.

PROTECTING PATIENT SAFETY
ACRO member companies are committed to ensuring the safety of research participants and patients. Protecting patient and participant safety is just as important as ensuring the integrity of data gleaned from clinical research trials. As we continue to lead the globalization of research trials and drug development, ACRO members:
- Promote the development of strong regulatory oversight in countries where clinical trials take place.
- Invest heavily in clinical staff training, education, advanced technologies and equipment. These investments not only facilitate the clinical research process but also improve and expand access to higher-quality healthcare.
- Lead efforts to promote and adhere to the International Council on Harmonization’s Good Clinical Practice guidelines for clinical trials. The ethical and legal codes governing medical practice also apply to clinical research trials, which is why most research trials are federally regulated with built-in safeguards to protect patients and participants.
ECONOMIC AND HEALTHCARE ENGINES
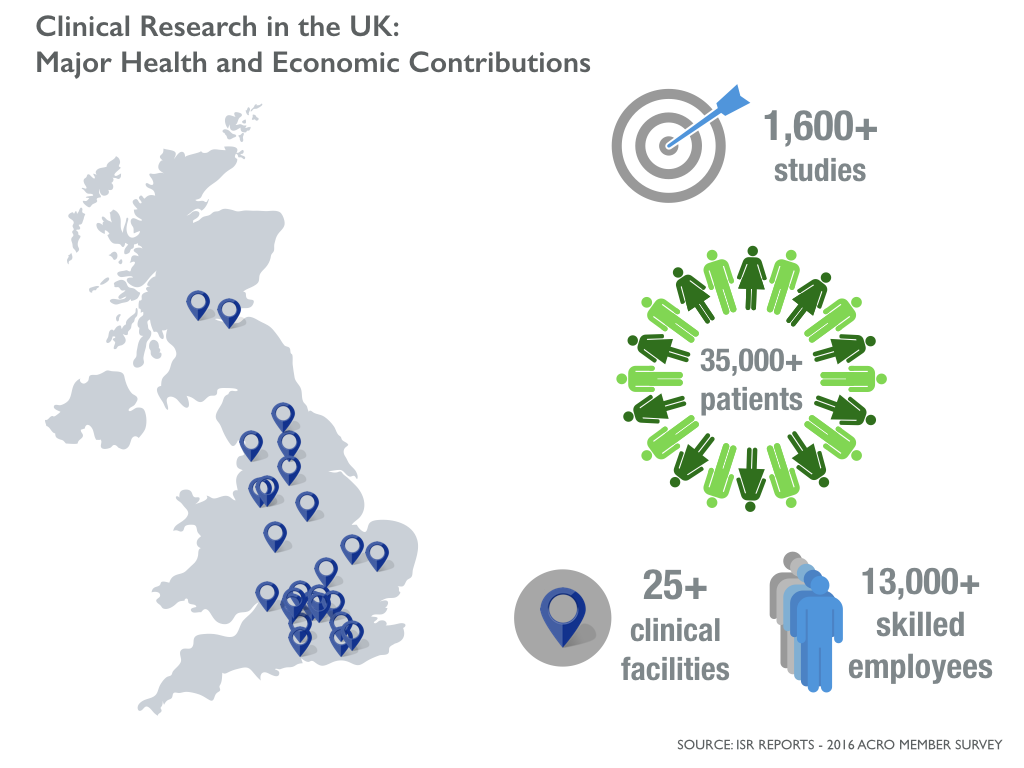
The clinical research industry touches lives and communities. Companies that conduct and support trials provide healthcare for patients, strengthening national health systems, while supporting a specialized workforce. In the UK, for example, ACRO member companies are a major source of employment, providing over 13,000 highly skilled jobs. ACRO members reach more than 35,000 patients in the UK through over 1,600 studies each year. These economic and health system impacts are an important aspect of clinical research.
Get the free factsheet!
Learn more about the steps in the drug development process and why clinical research and technology organizations are critical to medical innovation. Download it and share!

