RBQM Survey Summary Report
ACRO’s 6-year survey of member company CROs demonstrates how
risk-based approaches are increasingly used in clinical trials
Overview Ongoing Trials New Studies Adoption What’s Next Download the PDF
Overview
In early 2025, ACRO conducted its sixth consecutive landscape survey. The following report highlights ACRO’s key findings for the prior year. The purpose of the annual survey is to evaluate adoption levels among ACRO member companies in order to improve our understanding of how risk-based quality management is being adopted across the clinical trial industry.
In 2024, 96% of clinical trials included at least one RBM or RBQM component, a significant increase from 53% in 2019.
In 2019, nearly half of clinical trials depended on traditional methods. Today, that figure has dwindled to just 4% as more innovative approaches are being used.
As clinical trials running in traditional operating models reach their planned completion, the data shows that for new trials CROs are increasingly adopting more efficient risk-based monitoring operating models. Usage of traditional methods was sliced in half between 2019 and 2020, and again from 2020 to 2021.
A Closer Look at the Studies in Our Dataset
The 2024 RBQM landscape survey includes 3,758 outsourced studies from 7 CRO member companies. The majority of studies in this group are considered small (defined as <300 participants), but the organizations involved represent a broad range of biopharmaceutical companies of all sizes. The following charts are representative of the activities across the clinical trial industry, including the sponsors’ overall adoption of RBQM.
Small Studies <300 participants; Mid-size Studies between 300-999 participants; Large/Mega Studies = 1,000+ participants
Ongoing Studies
Adoption of RBQM Components
The following graphs show how RBQM components have been adopted in ongoing clinical trials each year between 2019 and 2024. The data was collected from ACRO Member CROs as part of the organization’s annual RBQM landscape survey.
Highlights
ACRO believes that initial and ongoing risk assessments are happening in every study. While the data shows 89-93% in recent years, we believe that many sponsors are bringing risk assessments in-house; this survey only examines the services that CROs provide.
Following the COVID-19 global pandemic, the data shows sizable jumps in centralized monitoring and off-site or remote monitoring. Despite a slight dip in 2024, adoption of these components far exceeds pre-pandemic figures.
Observations about Reduced SDR vs. SDV
The data shows that sponsors are more likely to reduce source data validation (SDV) than they are to reduce source data review (SDR). It appears that SDR is a comfort factor for studies that are implementing centralized monitoring. The majority of ongoing clinical trials incorporate risk assessments, with nearly half also implementing key risk indicators (KRIs), central monitoring, and off-site remote monitoring.
Despite these advancements, an unexpected number of trials still rely on 100% source data review (SDR) and source data verification (SDV). This highlights a significant opportunity for improvement and a need to rethink traditional approaches across all trial phases and therapeutic areas—especially as increasing study complexity calls for broader adoption of centralized monitoring strategies.
ACRO members believe that as centralized monitoring becomes more generally accepted as ‘standard practice’, comparable reductions in SDR and SDV will occur in parallel.
New Study Starts
Adoption of RBQM Components
ACRO ran the same analysis on new study starts each year between 2019 and 2024. Observing newly started studies could shed light on the trendline for operation models in studies begun this year.
Highlights
- Roughly half of new studies outsourced to CROs utilize risk assessments, KRIs, centralized monitoring, and remote monitoring.
- There was a small decrease in the use of KRIs and remote monitoring. Refinement of centralized monitoring strategies including study specific analyses and more efficient recognition of problems might explain this decrease.
- ACRO’s dataset shows that industry adoption of RBQM components has steadily grown from 2019 to 2024. However, we are seeing a decline in the adoption of certain components, namely reduced SDR. We’re still seeing 100% SDR/SDV on most studies.*
- In large/mega-sized studies (defined as > 1,000 participants) started in 2024, 100% SDR is being used 82% of the time and 100% SDV is being used 30% of the time. This is costing the industry a lot of time, capital, and human resources for little return.
Risk Proportionality and Centralized Monitoring
The FDA has reiterated the concept of risk proportionality, which focuses resources on high-risk areas while avoiding unnecessary efforts in low-risk areas. Centralized monitoring does just that: enables early detection of issues, improves data quality, increases patient safety, and reduces expending unnecessary resources.
Despite this, there is an apparent hesitancy, stemming from risk aversion, lack of trust, and fear of missing adverse events, to move away from traditional trial elements like SDR and SDV.
The stakes are high in a clinical trial, and companies want to ensure they are not missing anything. However, experience suggests 100% SDR/SDV leaves more room for errors and opportunities for mistakes. It can also create logistical challenges for CROs and sponsors that cost valuable time and money.
* Differing functional service provider (FSP) models are commonly used by sponsors, and this may be contributing to the high levels of 100% SDR/SDV that we are seeing in our dataset. If a sponsor deploys a FSP strategy and contracts with multiple vendors or CROs on a given study, this may introduce an additional level of risk due to the need for the different vendors to closely coordinate their activities in deployment of a successful RBQM strategy. To mitigate this risk, sponsors may be more inclined to include 100% SDR/SDV as a back-up when outsourcing in this model. ACRO believes that RBQM should be implemented in a holistic end-to-end manner in all outsourcing models, improving monitoring of a trial and data quality.
Adoption
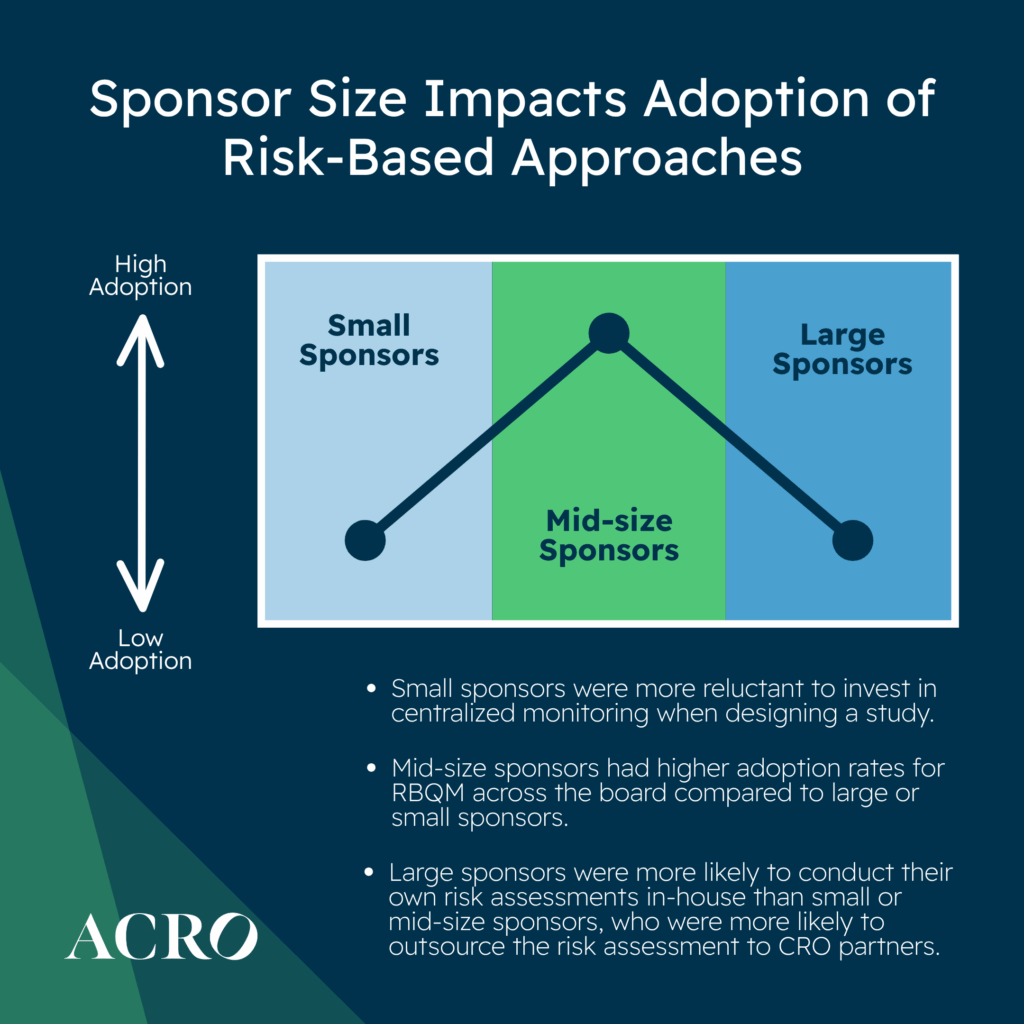
Does Adoption Differ by Sponsor Size?
When looking at new study starts, mid-size sponsors had higher adoption rates across the board compared to large or small sponsors. ACRO’s data shows that large sponsors were more likely to conduct their own risk assessments in-house than small or mid-size sponsors, who were more likely to outsource risk assessments to CRO partners.
Mid-size sponsors were more likely to reduce SDR as compared to small and large sponsors. Mid-size and large sponsors were more likely to reduce SDV as compared to small sponsors.
In our experience, smaller sponsors are generally more reluctant to invest in centralized monitoring when designing a study, but we believe by doing so, the cost of monitoring could drastically be reduced through reductions in SDR/SDV and on-site monitoring.
Does Adoption Differ by Study Size?
When looking at new study starts, ACRO’s data shows that small and mid-size studies (defined as 300-999 participants) were less likely to outsource risk assessments. Mid-size and large/mega studies were more likely to implement KRIs, centralized monitoring and reduction of SDV as compared to small studies. Mid-size studies were more likely to utilize QTLS, off-site or remote monitoring, and reduction of SDR as compared to small and large/mega studies.
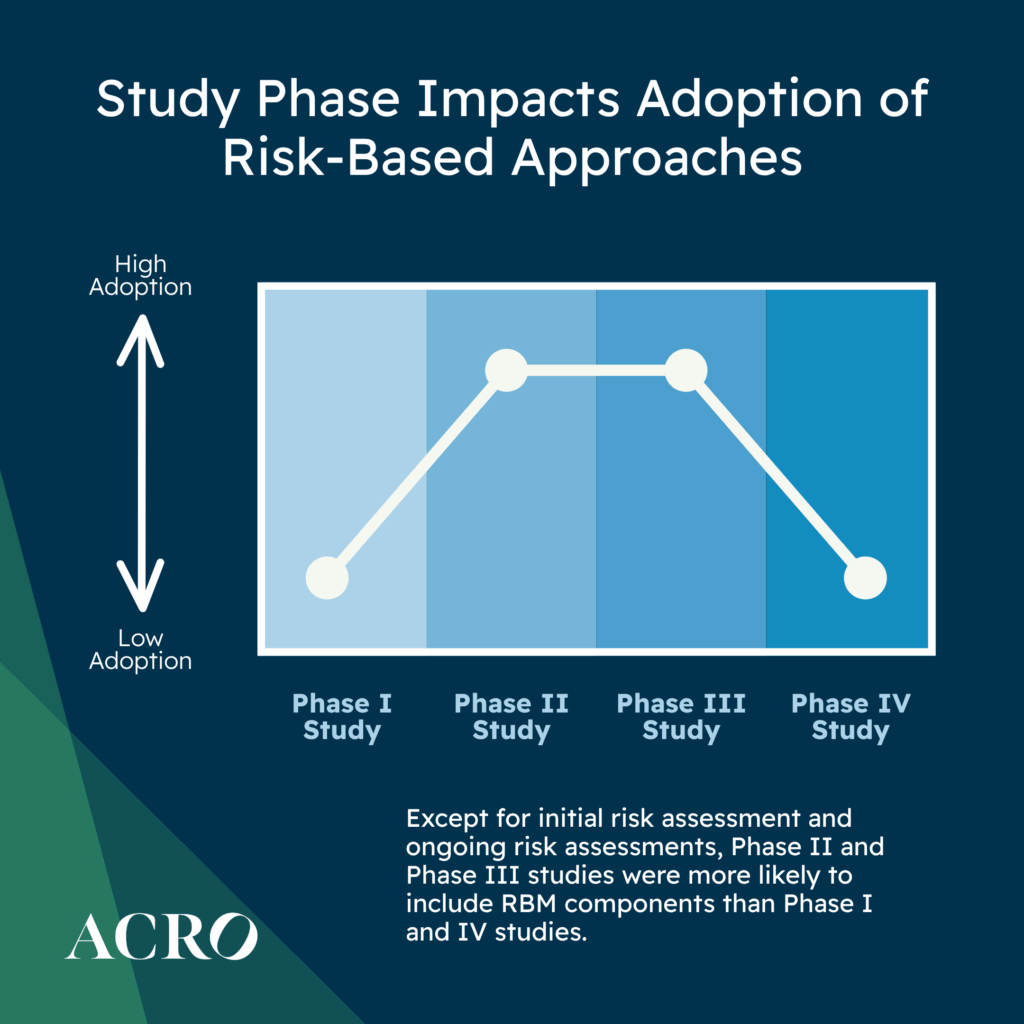
Does Adoption Differ by Phase?
For new study starts, ACRO’s data shows that except for initial and ongoing risk assessments, Phase II and Phase III studies were more likely to include RBQM components as compared to Phase I and IV studies. Phase II studies were more likely to incorporate off-site monitoring. Phase III studies were more likely to utilize QTLs, KRIs, centralized monitoring, and reduced SDR/SDV.
Given the wide variation in adoption across study size and phase, it is worth considering that certain RBQM components are applied on a fit-for-purpose basis. This would account for the variation we see.
The number of data sources1 in clinical studies is ever expanding due to increased utilization of electronic patient-reported outcomes (ePRO), electronic clinical outcome assessments, (eCOA), wearable devices, etc. According to a 2022 study led by Tufts CSDD in collaboration with a working group of pharmaceutical companies and CROs, there were more than 3.5 million data points in Phase III protocols alone.2
The onsite, manual monitoring methods associated with traditional monitoring are limited in scope and will not be able to keep pace with data volume and complexity, necessitating increased adoption of RBQM. 100% SDR/SDV is no longer feasible with the volume of data in a modern trial.
An analysis done by the Society for Clinical Data Management indicates that upwards of 70% of data volume3 is not coming from EDC, but rather from other sources (e.g., lab data). As a result of more direct participant or clinician data sources as well as technological enhancements to connect eSource and electronic Health Records directly to EDC less transcription activity is required by sites. As the industry moves away from systems in which data is manually transcribed, and moves towards direct data sources, the need for SDV will be significantly reduced if not eliminated entirely.
Advancements in artificial intelligence (AI) are opening new opportunities to maximize accuracy and efficiency in clinical data review. In the future, AI will be increasingly employed in RBQM to improve the efficiency and accuracy of data collection and monitoring , especially data volume and complexity intensifies. As organizations continue to implement and expand their RBQM approaches, they should take into consideration how AI and Machine Learning (ML) can be leveraged. The FDA is leading the way by fully embracing AI, and the industry should follow suit.
1 Society for Clinical Data Management, 2019, The Evolution of Clinical Data Management into Clinical Data Science: A Reflection Paper on the Impact of the Clinical Research Industry Trends on Clinical Data Management , https://scdm.org/wp-content/uploads/2024/03/2019_Evolution-of-CDM-to-CDS-Part-1-Drivers.pdf. Accessed 12 June 2025.
2 Getz, K., Smith, Z. & Kravet, M. Protocol Design and Performance Benchmarks by Phase and by Oncology and Rare Disease Subgroups. Ther Innov Regul Sci 57, 49–56 (2023). https://doi.org/10.1007/s43441-022-00438-5
3 Society for Clinical Data Management, 2019, The Evolution of Clinical Data Management into Clinical Data Science: A Reflection Paper on the Impact of the Clinical Research Industry Trends on Clinical Data Management, https://scdm.org/wp-content/uploads/2024/03/2019_Evolution-of-CDM-to-CDS-Part-1-Drivers.pdf. Accessed 12 June 2025.
