In 2024, ACRO’s RBQM Working Group conducted the fifth consecutive year of its annual risk-based quality management (RBQM) landscape survey. ACRO’s newly released report, Risk-Based Monitoring in Clinical Trials: A Five-Year Summary Report, highlights key trends and findings from the survey.
The aim of the landscape survey is to provide an answer to the interest that both ACRO members and global regulators have demonstrated in understanding how risk-based monitoring (RBM), and the larger framework of RBQM, are being adopted across the clinical trial industry. Conversations with the FDA helped to inform survey content and development.
Prevalence of Risk-Based Components in Clinical Trials
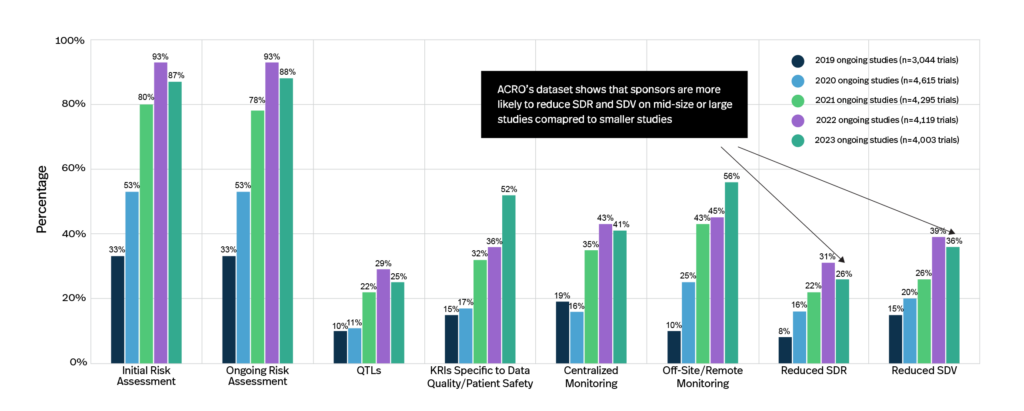
ACRO’s dataset shows that industry adoption of RBM and RBQM components has steadily grown from 2019 to 2023. However, centralized monitoring is still being underutilized (41% of clinical trials in 2023 utilize it). With millions of data points across multiple sources, it is imperative that the industry aggregates data and deploys centralized monitoring more broadly in order to improve issue detection and safety observations.
See the complete landscape survey results and read the report:
